Abstract
Background: Primary immune thrombocytopenia (ITP) is an acquired autoimmune disorder that results from accelerating platelet clearance, destruction, and production. Front-line therapy for newly diagnosed ITP includes corticosteroids, intravenous immune globulin, or anti-D immunoglobulin. However, after these single-agent therapies, relapses will occur in half of patients. We previously reported the safety, feasibility, and efficacy of the combination of dexamethasone, low-dose rituximab, and the thrombopoietin receptor agonist (TPO-Ra) eltrombopag as front-line treatment in newly diagnosed ITP. Romiplostim, another TPO-Ra is approved by the FDA for patients with chronic ITP. However, the safety, tolerability, and efficacy of romiplostim combined with low-dose rituximab, and high-dose dexamethasone in newly diagnosed ITP remain unknown.
Objective: Our primary objectives were safety, and tolerability. Secondary objectives were initial response and relapse incidence.
Methods: An open-label, single-arm study was performed in Hospital Universitario "Dr. José Eleuterio González" in Monterrey, Mexico (Clinical trials.gov NCT04588194). Eligible patients were newly diagnosed ITP patients, treatment-naïve, ≥18 years, with a platelet count ≤30×10 9/L. Patients were excluded if they had an active infection, pregnancy, or malignant disease. The treatment regimen was romiplostim (2 mcg/kg weekly, four doses), low-dose rituximab (100 mg weekly, four doses) and high-dose dexamethasone (40 mg PO, days 1-4). Dexamethasone was allowed up to 3 cycles. Partial (PR) and complete responses (CR) were defined as an increase in platelet counts ≥30×10 9/L (at least a doubling of the baseline count) and ≥100×10 9/L, respectively.
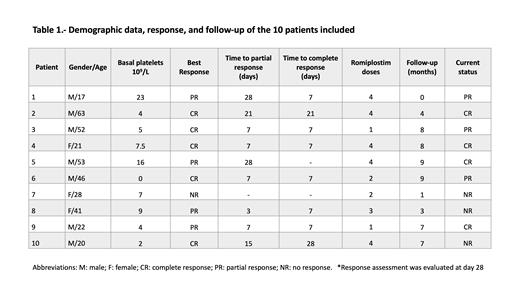
Results: We included ten consecutive patients. The median age was 34.5 years (range, 17-63). Seven patients were men (70%). The median platelet account at diagnosis was 6x10 9/L (range 0-23), and median follow-up was 180 days (range 30-270). The median number of romiplostim doses was 3.5 (range 1-4), and three (30%) patients required dose adjustment due to thrombocytosis. All but one patient achieved response (CR or PR) at a median of 7 days (range 7-28). Five patients (50%) achieved CR at 28 days of treatment, and four patients (40%) PR. No significant adverse effects have occurred during treatment; one patient presented grade 1 myalgia and the other a grade 2 soft tissue infection. Five patients (50%) relapsed during follow-up. Recently, four (40%) patients remain in CR and three (30%) in PR.
Conclusion: The combination of romiplostim, low-dose rituximab, and high-dose dexamethasone was safe and effective. This "total therapy" approach was associated with minimum side effects and rapid initial response. Prospective validation in a larger sample is needed.
Gomez-Almaguer: Janssen: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau; Bristol-Myers-Squibb: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau.
Romiplostim in firs-line therapy for immune thrombocytopenia